As announced by the UK government on 24 August 2021, the transition period for CE marked products has been extended by one year.
This means that CE-marked products may now be placed on the market in the United Kingdom until January 1st, 2023, despite Brexit. Only from 2023 will it be obligatory to bring exclusively UKCA (UK Conformity Assessed) marked products onto the market. Originally, the deadline was set for January 1st, 2022. (On UKCA marking, also read the report in the Holz-Zentralblatt of 13 August 2021 on our web seminar COMPETENCE MEETS COOPERATION with Chris Miles from UL UK on UKCA marking, which took place on June 22nd, 2021, here).
Products placed on the market in Northern Ireland are exempt from the regulation: for these, the CE marking will still be recognised after the deadline if the products also bear a UKNI (UK North Ireland) marking.
For products from the medical industry, the deadline is July 1st, 2023.
Go to original post by UK government from August 24th, 2021
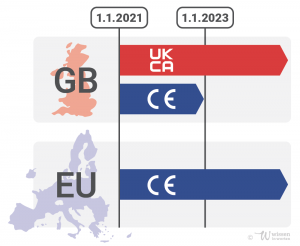
Diagram: Karin Roth for eco-INSTITUT
Source: https://www.gov.uk/government/news/businesses-given-more-time-to-apply-new-product-safety-marking (las accessed August 31st, 2021)